Announcement of the State Intellectual Property Office on Amending "Guidelines for Patent Examination" (No. 391)
2025-04-30
"Guidelines for Patent Examination" had been decided to be amended by CNIPA, which is hereby issued and will take effect as of the date of January 15, 2021.
CNIPA decided to make modifications to Guidelines for Patent Examination as follows:
First Modifications to Section 3.5, Chapter 10, Part Ⅱ
3.5 Supplementary Experimental Data
3.5.1 Principles for Examination
Whether the description is sufficiently disclosed or not is judged on the basis of the content recorded in the initial description and claims.
For the experimental data submitted after the date of filing by the applicant for the purpose of meeting the requirements of Rule 22.3 and Rule 26.3 in the Patent Law, the examiners shall take it into examination. The technical effects proved by the supplementary experimental data shall enable a person skilled in the art to obtain it from the disclosure contained in the patent application.
3.5.2 Supplementary Experimental Data of Drug Patent Application
In accordance with the examination principle of Section 3.5.1 in this chapter, here are examples concerning drug patent application.
[Example 1]
Claim requested for protection of compound A, the description recorded its preparation of embodiment, hypotensive effect and experimental method of measuring the activity of hypotensive action without recording experimental result data. In order to prove that the description is sufficiently disclosed, the applicant submitted antihypertensive effects data of compound A. As for those skilled in the art, according to the record of initial application document, the hypotensive effect of compound A has been disclosed, the technical effect that experimental data submitted try to prove can be obtained from the patent application document. It shall be noticed that the said data should be examined during the examination of inventive step.
[Example 2]
Claim requested for protection of the compounds of general formula Ⅰ, the description recorded general formula Ⅰ and its preparation method, embodiment of multiple specific compounds such as A, B and others in general formula Ⅰ, as well as antitumor effect of general formula Ⅰ, the experimental method of measuring the activity of antitumor action and its experimental result data which recorded that the IC50 value of tumor cells ranges from 10 to 100nM. In order to prove that the claim is creative, the applicant submitted contrast experiment data, showing the IC50 value of compound A is 15nM, which is 87nM of compound in document 1. As for those skilled in the art, according to the record of initial application document, compound A and its antitumor effect have been disclosed, the technical effect that experimental data submitted aims to prove can be obtained from the patent application document. What shall be noted is that the examiners need to analyze whether the technical solution claiming for protection meets the requirements of inventive step combining the data flied after the examination.
Second Modifications to Section 4.2.3 of Chapter 10, Part Ⅱ
In the last paragraph in Section 5.1, Chapter 10, Part Ⅱ of Guidelines for Patent Examination, “The composition shall be drafted as the function-defining or use-defining type” is revised as “The composition usually need to be drafted as the function-defining or use-defining type”, and “In certain fields, such as the field of alloys, the intrinsic property and/or use of the invented alloy usually shall be specified. Most pharmaceutical claims shall be drafted as the use-defining type.” is revised as “In certain fields, such as the field of alloys, the intrinsic performance and/or use of the invented alloy usually shall be specified. Most pharmaceutical claims shall be drafted as the use-defining type. ”
No revision of other contents in this section.
Third Modifications to Section 5.1 of Chapter 10, Part Ⅱ
Item (1) in Section 5.1, Chapter 10, Part Ⅱ of Guidelines for Patent Examination is revised as:
(1) For a compound claimed in an application, if a citation recorded structure information of the compound, such as the chemical name, the molecular formula (or structural formula), making those skilled in the art believe that the compound has been disclosed, then the said compound does not involve novelty, unless the applicant can provide evidence to verify that the compound is not available before the date of filing.
If it is insufficient to identify the similarities and differences between the claimed compound and the compound disclosed in the citations in accordance with the structure information recorded in the citations, but after comprehensively considering other information recorded in the said document, including physicochemical parameters, method of preparation and effect experimental data, those skilled in the art have reasons to presume that two compounds have the same essence, then the claimed compound does not involve novelty, unless the applicant can provide evidence to prove that there has been a real structural difference.
No revision of other contents in this section.
Fourth Modifications to Section 6.1 of Chapter 10, Part Ⅱ
Section 6.1, Chapter 10, Part Ⅱ of Guidelines for Patent Examination is revised as:
6.1 Inventive step of Compound
(1)To judge the inventive step of compound invention shall determine the structural difference between the compound claiming for protection and the compound which is most similar to the prior techniques, and on the basis of the use and/or effect obtained by this structural transformation, make sure the technique issues solved by the invention, based on the above, judge whether the prior art has offered enlightenment of solving technical problems through this structural transformation.
It shall be noted that if a person skilled in the art can solve all mentioned technical issues and obtain compound requesting for protection with the structural transformation through logical analysis and inference as well as limited experiments, then the prior art is deemed to have technical enlightenment.
(2)The use and/or effect brought by the structural transformation carried out on the compound that is the closest to prior art by the invention may be obtaining use different from the known compounds, or the improvement on some effects of the known compounds. When judging the inventive step of a compound, if the change of use and/or improvement of effect is unpredictable, which reflects the compound requesting for protection is not obvious, then its inventive step shall be approved.
(3)It is need to explain that when judging the inventive step of a compound invention, if the effect of a technical solution claiming for protection is caused by something known and inevitable, the technical solution does not involve inventive step. For example, an insecticide A-R is in the prior art, wherein, R is C1-3 alkyl, and it has been pointed out in the prior art that the effectiveness of insecticide is improved with the increase of the number of atom in the alkyl. If the insecticide in an application is A-C4H9, the effectiveness has been obviously improved in comparison of the prior art. The application does not involve inventive step because it has been pointed out in the prior art that the improved effectiveness of the insecticide is inevitable.
(4)Examples of Inventive step Judgement
[Example 1]
Prior art:
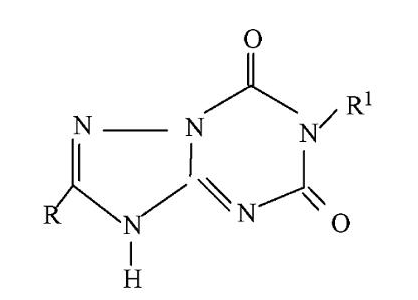
(Ⅰa)
Application:

(Ⅰb)
(Ⅰb) and (Ⅰa) have different core structures but both are used for the same purpose. Those skilled in the art generally believe that compounds with similar structures have the same or similar uses, and similar structures generally refer to that the compounds have the identical basic core structure or basic rings. In the prior art, there is no technical enlightenment that the basic ring of (Ⅰa) is modified to obtain (Ⅰb) and the use remains unchanged, so (Ⅰb) involves inventive step.
[Example 2]
Prior art: H2N-C6H4-SO2NHR1 (Ⅱa)
Application: H2N-C6H4-SO2-NHCONHR1 (Ⅱb)
(Ⅱb) is (Ⅱa) with an insertion of -CONH- in the (Ⅱa) NHR1 structural fragment. The use of (Ⅱb) and (Ⅱa) is completely different. (Ⅱa) Sulfonamide is an antibiotic, and (Ⅱb) Sulfonylurea is an antidiabetic drug. The (IIb) involves inventive step because a person skilled in the art has no motive to transform R1 in antibiotics into CONHR1 to obtain antidiabetic drugs, so (Ⅱb) involves inventive step.
[Example 3]
Prior art: H2N-C6H4-SO2NHCONHR1(Ⅲa)
Application: H3C-C6H4-SO2NHCONHR1(Ⅲb)
Amino-sulfonylurea (Ⅲa) and methyl-sulfonylurea (Ⅲb) are only different from the structure of NH2 and CH3. Both are antidiabetic drugs with equivalent effects. Compared with (Ⅲa), (Ⅲb) provides another anti-diabetic drug in the art. Since NH2 and CH3 are classical isosteres of valence electron, those skilled in the art have the motive to carry out this isostere replacement in order to obtain the same or equivalent antidiabetic activity, (Ⅲb) does not involve inventive step.
[Example 4]
Prior art:
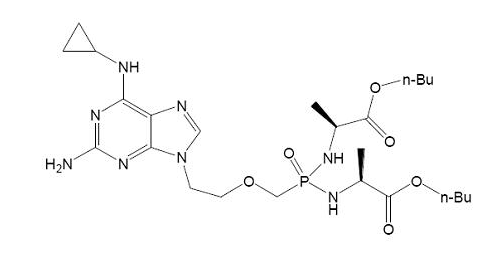
(Ⅳa)
Application: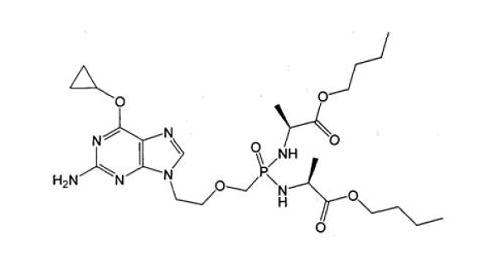
(Ⅳb)
The difference between compound (Ⅳb) and compound (Ⅳa) is only the replacement of -NH- by -O- at the 6-position of the purine. Although -O- and -NH- are classical isosteres well-known in the art, the cancer cell growth inhibitory activity of (Ⅳb) enhances about 40 times higher than that of (Ⅳa), and (Ⅳb) obtains unexpected technical effect in comparison of (Ⅳa), which indicates that (Ⅳb) is unobvious, so (Ⅳb) involves inventive step.
[Example 5]
Prior art:
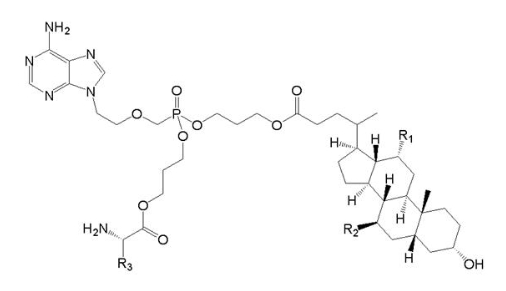
(Ⅴa)
Wherein, R1=OH, R2=H and R3=CH2CH(CH3)2.
Application:
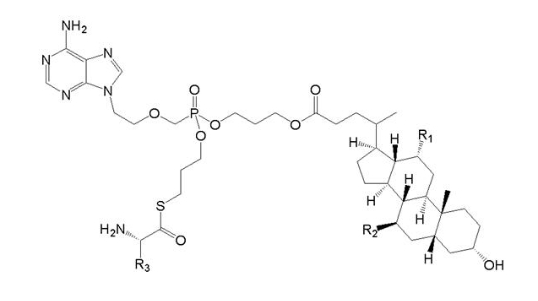
(Ⅴb)
Wherein, R1 and R2 are chosen from H or OH, R3 from C1-6 alkyl, also including specific compound (Ⅴb1)with structure of R1=OH, R2=H and R3=CHCH3CH2CH3. In addition, the anti-HBV activity of (Ⅴb1) is obviously better than that of (Ⅴa).
When claiming for protection of (Ⅴb) general formula compound, (Ⅴb) and (Ⅴa) only differ in the linked atom between phosphoryl alkyl and amino acid residue, where -S- is for (Ⅴb), and -O- is for (Ⅴa). Compared with (Ⅴa) , (Ⅴb) general formula compound provides another anti-HBV drug in the art. Due to the similar property of -S- and -O-, for the purpose of obtaining other drugs with anti-HBV activity as well, those skilled in the art has the motive to replace and obtain (Ⅴb) general formula compound, so (Ⅴb) does not involve inventive step.
When claiming for protection of (Ⅴb1) specific compound, the difference between (Ⅴb1) and (Ⅴa) involves not only the said above linked atom, but also the substituent at the R3 position, and anti-HBV activity of (Ⅴb1) is obviously better than (Ⅴa). There is no technical enlightenment on enhancing anti-HBV activity by the said above structural transformation in the prior art, so (Ⅴb1) involves inventive step.
Fifth Modifications to Section 9.2.1 of Chapter 10, Part Ⅱ
In the Item(4) of Section 5.1, Chapter 10, Part Ⅱ of Guidelines for Patent Examination, “including the Center for General Microorganism of the Administration Committee of the China Microbiological Culture Collection (CGMCC) based in Beijing and the China Center for Type Culture Collection (CCTCC) based in Wuhan.” is revised as “including the Center for General Microorganism of the Administration Committee of the China Microbiological Culture Collection (CGMCC) based in Beijing, the China Center for Type Culture Collection (CCTCC) based in Wuhan and Guangdong Province Center for Microbiological Culture Collection based in Guangzhou.”
No revision of other contents in this section.
Sixth Modifications to Section 9.3.1.7 of Chapter 10, Part Ⅱ
Section 9.3.1.7, Chapter 10, Part Ⅱ of Guidelines for Patent Examination is revised as:
9.3.1.7 Monoclonal Antibody
A claim directed to a monoclonal antibody may be defined by architectural feature and also may be defined by specifying hybridoma which produces it.
[Example]
(1) A monoclonal antibody against antigen A includes amino acid sequences like VHCDR1, VHCDR2 and VHCDR3 as shown in SEQ ID NO:1-3, and that such as VLCDR1, VLCDR2 and VLCDR3 as shown in SEQ ID NO:4-6.
(2) A monoclonal antibody against antigen A, produced by a hybridoma having CGMCC Deposit No. xxx.
Seventh Modifications to Section 9.4.2 of Chapter 10, Part Ⅱ
1 Three paragraphs are newly added into Section 3.5, Chapter 10, Part Ⅱ of Guidelines for Patent Examination as follows:
The judgement of inventive step of inventions in the field of biotechnology also includes judging whether or not the invention has excellent substantive features and prominent improvement. During the process of judgement, it is need to determine the distinction and features between the invention and closest prior art in accordance with specific limited contents of different protection themes, then confirm the technical problems actually solved by the invention on the basis of the technical effect that can be achieved by the said distinction and features in the invention, finally judge whether the prior art has provided technical instructions overall. Based on the above, it can be concluded that whether the invention is obvious in relation to the prior art.
The invention-creations in the field of biotechnology involve protection themes of different levels such as biomacromolecule, cell, microoraganism, etc. In the way of characterizing these protection themes, there are also special ways including deposit number of biological materials except for the common ways like structure and composition. To judge inventive step, the structural discrepancy and the ties of consanguinity between the invention and the prior art, and the predictability of technical effect shall be taken into consideration.
Hereinafter shows certain specific circumstances in the inventive judgement of various protection themes in this field.
2 Item(1) in Section 9.4.2.1, Chapter 10, Part Ⅱ of Guidelines for Patent Examination is revised as:
(1) Gene
Compared with a known protein, if a protein encoded by a structural gene has a different amino acid sequence and a different type or improved performance, and the prior art does not involve the technical enlightenment on the above performance change brought by the sequence difference, then the gene invention encoding the protein involves inventive step.
If the amino acid sequence of a protein is known, then an invention of a gene encoding the protein does not involve inventive step. If a protein is known but its amino acid sequence is not, an invention of a gene encoding the said protein does not involve inventive step as long as a person skilled in the art may easily determine the amino acid sequence when submitting the application. However, under the above two circumstances, if compared with other genes encoding the said protein with a different base sequence, the gene with a specific base sequence has an unexpected technical effect for those skilled in the art, then the invention of that gene involves inventive step.
If the claimed structural gene of an invention is a naturally available mutant structural gene of a known structural gene, and the said structural gene claiming for protection and the known structural gene originates from the same species with the same properties and functions as well, then the invention does not involve inventive step.
3 Item (2) Polypeptide or Protein is added into Section 3.5, Chapter 10, Part Ⅱ of Guidelines for Patent Examination as follows:
(2) Polypeptide or Protein
If the claimed polypeptide or protein of an invention differs in amino acid sequence in relation to the known polypeptide or protein, and has different types or improved properties, and the prior art does not provide technical enlightenment of the above mentioned performance changes brought by the sequence difference, then the invention of the polypeptide or protein involves inventive step.
4 Item(2) Recombinant Vector in Section 9.4.2.1, Chapter 10, Part Ⅱ of Guidelines for Patent Examination is revised as (3) Recombinant Vector, and one paragraph is inserted before the original content as follows:
If the invention improves the performance of the recombinant vector aiming at the structural modification of a known vector and/or inserted gene, and the prior art does not provide technical enlightenment with the above-mentioned structural modification to improve the performance, then the invention of the recombinant vector involves inventive step.
5 Item(3) Transformant in Section 9.4.2.1, Chapter 10, Part Ⅱ of Guidelines for Patent Examination is revised as (4) Transformant, and one paragraph is inserted before the original content as follows:
If the invention improves the performance of the transformant aiming at the structural modification of a known host and/or inserted gene, and the prior art does not provide technical enlightenment with the use of the above-mentioned structural modification to improve the performance, then the invention of the transformant involves inventive step.
6 Item (4) Fused Cell in Section 9.4.2.1, Chapter 10, Part Ⅱ of Guidelines for Patent Examination is revised as (5) Fused Cell:
7 Item (5) Monoclonal Antibody in Section 9.4.2.1, Chapter 10, Part Ⅱ of Guidelines for Patent Examination is revised as (6) Monoclonal Antibody, and the content is overall revised as:
If the antigen is known, the monoclonal antibody of the antigen characterized by structural features is obviously different from the known monoclonal antibody in the key sequence determining function and use, and the prior art does not supply the technical instruction of the monoclonal antibody obtaining the above-mentioned sequence, and the monoclonal antibody can produce beneficial technical effects, then the invention of the said monoclonal antibody involves inventive step.
If the antigen is known, and it is clear that the antigen is immunogenic (for example, it can be known that the antigen is obviously immunogenic due to the fact that the polyclonal antibody of the antigen is known or the antigen is a macromolecular polypeptide), then the invention of monoclonal antibodies just limited with the said antigen does not involve inventive step. However, if the invention is further limited by the hybridoma of a monoclonal antibody secreting the said antigen, and thus produces an unexpected effect, then the invention of that monoclonal antibody involves inventive step.
No revision of other contents in this section.
This Decision shall take into effect on January 15, 2021.

